This is an informational page for patients to learn facts about a specific recall. Click the links below to see if your implant is affected by a recall.
US Exactech Recall Information
2025
Important Patient Information Regarding the Equinoxe Shoulder System
About the Equinoxe® Shoulder System:
There are two types of shoulder replacement.
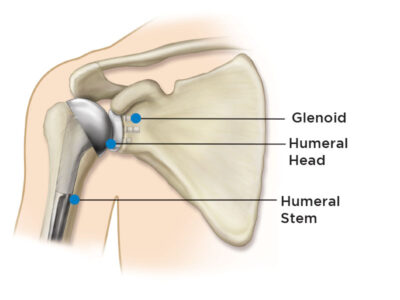
Anatomic Shoulder Replacement
In this procedure, the surgeon will remove the damaged bone and cartilage. The head of the humerus is then removed and a metal stem is placed into the humeral canal. This provides a stabilizing anchor for the head.
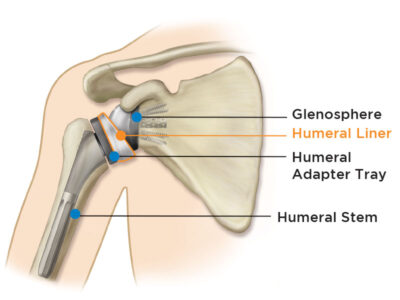
Reverse Shoulder Replacement
In this procedure, the anatomy of the shoulder is reversed by attaching a metal ball (glenosphere) to the glenoid and the plastic socket (humeral liner) to the upper humerus. A reverse shoulder replacement empowers your deltoid to become the main functioning muscle in the absence of a healthy rotator cuff.
This information applies to patients who received an Equinoxe Reverse Shoulder replacement. It was recently discovered that some Equinoxe Reverse Shoulder polyethylene humeral liners have an articular surface center of rotation slightly out of specification, up to 0.02 inches. Not all reverse shoulders were impacted.
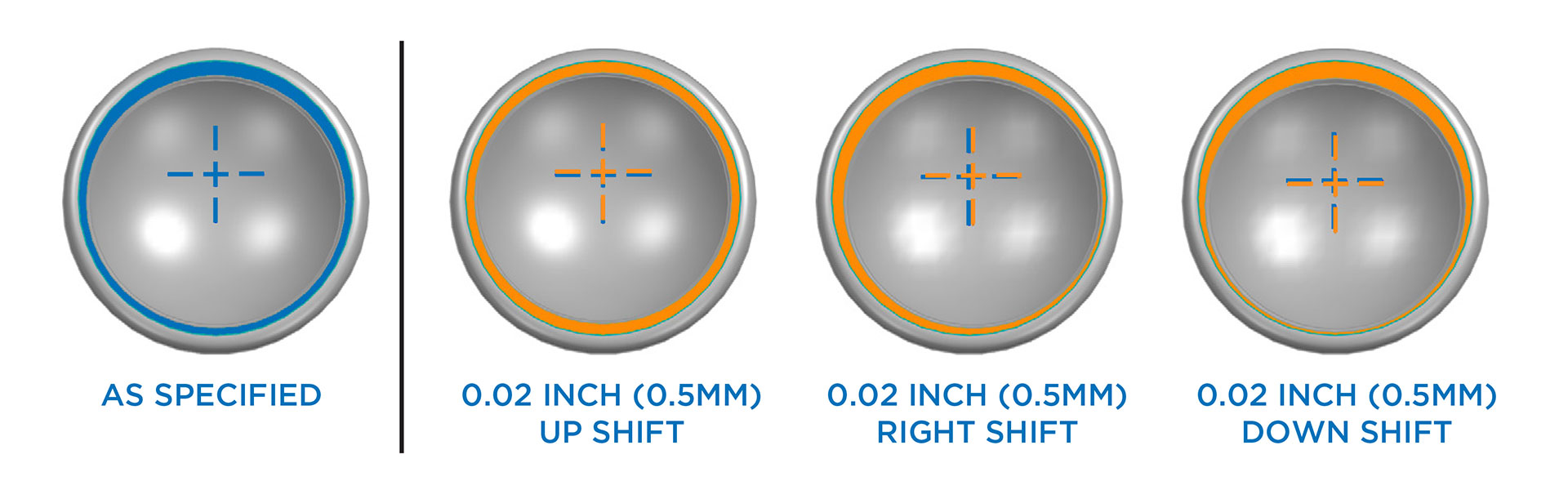
Reverse Humeral Liner surface shown 1) as specified and 2) with three possible manufacturing shifts of the center of rotation. Blue indicates specified center of rotation, orange indicates shifted position.
Due to this deviation from the designed center of rotation, we are conducting a voluntary medical device recall of specific humeral liners.
If your implant is within the scope of this recall, this does not mean that the shoulder component needs to be replaced.
The dimensional shift was identified through Exactech’s internal quality inspection. It may slightly decrease active range of motion by up to 1 degree. There are no related complaints or adverse events reported.
Normal patient monitoring and follow-up per your surgeon’s standard of care is recommended. Revisions of well performing shoulders using “shifted” humeral liners is not recommended.
If you have questions, please contact humeralCOR@exac.com.
The following products are affected:
Scroll right to view the full table on mobile devices.
| Item Number | Product Description | Brand Name | 510K # | SE Date | UDI |
|---|---|---|---|---|---|
| 320-38-00 | REVERSE SHOULDER,38mm Humeral Liner,+0mm | Equinoxe | K063569 | 2/23/2007 | 10885862086655 |
| 320-38-03 | REVERSE SHOULDER,38mm Humeral Liner,+2.5mm | Equinoxe | K063569 | 2/23/2007 | 10885862086662 |
| 320-42-00 | REVERSE SHOULDER,42mm Humeral Liner,+0mm | Equinoxe | K063569 | 2/23/2007 | 10885862086693 |
| 320-42-03 | REVERSE SHOULDER,42mm Humeral Liner,+2.5mm | Equinoxe | K063569 | 2/23/2007 | 10885862086709 |
| 322-38-00 | REVERSE SHOULDER,38mm Humeral Liner,+0mm | Equinoxe | K223833 | 9/14/2023 | 10885862593832 |
| 322-38-03 | REVERSE SHOULDER,38mm Humeral Liner,+2.5mm | Equinoxe | K223833 | 9/14/2023 | 10885862593849 |
| 322-42-00 | REVERSE SHOULDER,42mm Humeral Liner,+0mm | Equinoxe | K223833 | 9/14/2023 | 10885862593917 |
| 322-42-03 | REVERSE SHOULDER,42mm Humeral Liner,+2.5mm | Equinoxe | K223833 | 9/14/2023 | 10885862593924 |
Previous
Polyethylene
This is to inform you of recent observations made regarding the clinical performance of Exactech polyethylene inserts. This communication relates to Exactech knee and ankle polyethylene liners sold in the United States. The product-specific information is listed in the links below.
Scroll right to view the full table on mobile devices.
| Product Line | Specific Brand Name | Total Units Sold Globally (2004-2.22.2022) |
|---|---|---|
| OPTETRAK® All-polyethylene Tibial Components (TKR) | 5078 | |
| 200-11-XX | OPTETRAK® All-polyethylene CR Tibial Components | 1 |
| 200-12-XX | OPTETRAK® All-polyethylene CR Tibial Components | 36 |
| 200-13-XX | OPTETRAK® All-polyethylene CR Tibial Components | 117 |
| 200-14-XX | OPTETRAK® All-polyethylene CR Tibial Components | 79 |
| 200-15-XX | OPTETRAK® All-polyethylene CR Tibial Components | 38 |
| 200-16-XX | OPTETRAK® All-polyethylene CR Tibial Components | 8 |
| 204-11-XX | OPTETRAK® All-polyethylene PS Tibial Components | 1097 |
| 204-12-XX | OPTETRAK® All-polyethylene PS Tibial Components | 1834 |
| 204-13-XX | OPTETRAK® All-polyethylene PS Tibial Components | 1277 |
| 204-14-XX | OPTETRAK® All-polyethylene PS Tibial Components | 528 |
| 204-15-XX | OPTETRAK® All-polyethylene PS Tibial Components | 61 |
| 204-16-XX | OPTETRAK® All-polyethylene PS Tibial Components | 2 |
| OPTETRAK® All-polyethylene Tibial Components (PKR) | 3428 | |
| 252-12-XX | OPTETRAK® All-polyethylene UNI Tibial Components | 1163 |
| 252-13-XX | OPTETRAK® All-polyethylene UNI Tibial Components | 1198 |
| 252-22-XX | OPTETRAK® All-polyethylene UNI Tibial Components | 524 |
| 252-23-XX | OPTETRAK® All-polyethylene UNI Tibial Components | 543 |
| OPTETRAK® Tibial Inserts (TKR) | 218944 | |
| 200-21-XX | OPTETRAK® CR TIBIAL INSERT | 5803 |
| 200-22-XX | OPTETRAK® CR TIBIAL INSERT | 24356 |
| 200-23-XX | OPTETRAK® CR TIBIAL INSERT | 20527 |
| 200-24-XX | OPTETRAK® CR TIBIAL INSERT | 9570 |
| 200-25-XX | OPTETRAK® CR TIBIAL INSERT | 3032 |
| 200-26-XX | OPTETRAK® CR TIBIAL INSERT | 270 |
| 200-50-XX | OPTETRAK® CR TIBIAL INSERT | 161 |
| 200-51-XX | OPTETRAK® CR TIBIAL INSERT | 4102 |
| 200-56-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 67 |
| 200-57-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 93 |
| 200-61-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 176 |
| 200-62-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 1012 |
| 200-63-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 1047 |
| 200-64-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 623 |
| 200-65-XX | OPTETRAK® CR TIBIAL SLOPE + INSERT | 204 |
| 200-71-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 148 |
| 200-72-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 906 |
| 200-73-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 1030 |
| 200-74-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 563 |
| 200-75-XX | OPTETRAK® CR TIBIAL SLOPE ++ INSERT | 218 |
| 204-21-XX | OPTETRAK® PS TIBIAL INSERTS | 10048 |
| 204-22-XX | OPTETRAK® PS TIBIAL INSERTS | 38886 |
| 204-23-XX | OPTETRAK® PS TIBIAL INSERTS | 32689 |
| 204-24-XX | OPTETRAK® PS TIBIAL INSERTS | 14623 |
| 204-25-XX | OPTETRAK® PS TIBIAL INSERTS | 4748 |
| 204-26-XX | OPTETRAK® PS TIBIAL INSERTS | 611 |
| 204-50-XX | OPTETRAK® PS TIBIAL INSERTS | 392 |
| 204-51-XX | OPTETRAK® PS TIBIAL INSERTS | 5516 |
| 204-91-XX | OPTETRAK "MOMB" NON-MOD MOLDED INSERT | 282 |
| 204-92-XX | OPTETRAK "MOMB" NON-MOD MOLDED INSERT | 864 |
| 204-93-XX | OPTETRAK "MOMB" NON-MOD MOLDED INSERT | 1167 |
| 204-94-XX | OPTETRAK "MOMB" NON-MOD MOLDED INSERT | 668 |
| 204-95-XX | OPTETRAK "MOMB" NON-MOD MOLDED INSERT | 176 |
| 208-21-XX | OPTETRAK® CC TIBIAL INSERT | 2996 |
| 208-22-XX | OPTETRAK® CC TIBIAL INSERT | 10026 |
| 208-23-XX | OPTETRAK® CC TIBIAL INSERT | 9143 |
| 208-24-XX | OPTETRAK® CC TIBIAL INSERT | 5175 |
| 208-25-XX | OPTETRAK® CC TIBIAL INSERT | 1824 |
| 208-51-XX | OPTETRAK® CC TIBIAL INSERT | 1136 |
| 224-21-XX | OPTETRAK® B-SERIES PS TIBIAL INSERT | 547 |
| 224-22-XX | OPTETRAK® B-SERIES PS TIBIAL INSERT | 1719 |
| 224-23-XX | OPTETRAK® B-SERIES PS TIBIAL INSERT | 1347 |
| 224-24-XX | OPTETRAK® B-SERIES PS TIBIAL INSERT | 453 |
| OPTETRAK® HI-FLEX® Polyethylene Tibial Inserts (TKR) | 51913 | |
| 244-20-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 226 |
| 244-21-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 6787 |
| 244-22-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 18921 |
| 244-23-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 15578 |
| 244-24-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 7662 |
| 244-25-XX | OPTETRAK® HI-FLEX® PS Polyethylene Tibial Inserts | 2739 |
| ARTHROFOCUS® Polyethylene Tibial Inserts (TKR) | 12 | |
| 256-12-XX | ARTHROFOCUS® Polyethylene Tibial Inserts | 6 |
| 256-13-XX | ARTHROFOCUS® Polyethylene Tibial Inserts | 6 |
| OPTETRAK® Custom Polyethylene Tibial Inserts (TKR) | 41 | |
| 900-06-XX | CUSTOM OPTETRAK® ANGLED PS INSERT | 6 |
| 900-08-XX | CUSTOM OPTETRAK® ANGLED PS INSERT | 6 |
| 900-23-XX | CUSTOM OPTETRAK® CC TIBIAL INSERT | 21 |
| 900-30-XX | CUSTOM OPTETRAK® CC TIBIAL INSERT | 4 |
| 900-33-XX | CUSTOM OPTETRAK® CC INSERT | 4 |
| OPTETRAK® LOGIC® Polyethylene Tibial Inserts (TKR) | 127998 | |
| 02-012-35-XXXX | OPTETRAK® Logic PS Tibial Inserts | 80506 |
| 02-012-44-XXXX | OPTETRAK® Logic PSC Tibial Inserts | 19323 |
| 02-012-47-XXXX | OPTETRAK® Logic CR Tibial Inserts | 9139 |
| 02-012-48-XXXX | OPTETRAK® Logic CR Slope + Tibial Inserts | 3083 |
| 02-012-49-XXXX | OPTETRAK® Logic CR Slope ++ Tibial Inserts | 2853 |
| 02-012-51-XXXX | OPTETRAK® Logic CRC Tibial Inserts | 10291 |
| 02-012-65-XXXX | OPTETRAK® Logic CC Tibial Inserts | 2803 |
| OPTETRAK® RBK® Polyethylene Tibial Inserts (TKR) | 33393 | |
| 264-21-XX | OPTETRAK® RBK PS Tibial Components | 2938 |
| 264-22-XX | OPTETRAK® RBK PS Tibial Components | 10994 |
| 264-23-XX | OPTETRAK® RBK PS Tibial Components | 11560 |
| 264-24-XX | OPTETRAK® RBK PS Tibial Components | 5738 |
| 264-25-XX | OPTETRAK® RBK PS Tibial Components | 2163 |
| TRULIANT® Tibial Inserts (TKR) | 38075 | |
| 02-022-35-XXXX | TRULIANT® PS Tibial Inserts | 20913 |
| 02-022-44-XXXX | TRULIANT® PSC Tibial Inserts | 6519 |
| 02-022-47-XXXX | TRULIANT® CR Tibial Inserts | 3095 |
| 02-022-48-XXXX | TRULIANT® CR Slope + Tibial Inserts | 504 |
| 02-022-49-XXXX | TRULIANT® CR Slope ++ Tibial Inserts | 335 |
| 02-022-51-XXXX | TRULIANT® CRC Tibial Inserts | 6709 |
| OPTETRAK® Logic RBK Tibial Components (TKR) | 10616 | |
| 02-012-38-XXXX | OPTETRAK® Logic RBK PS Tibial Components | 10616 |
| Vantage® Fixed-Bearing Polyethylene Liner Component (TAR) | 2959 | |
| 350-21-XX | Vantage® Fixed-Bearing Polyethylene Liner Component | 1422 |
| 350-22-XX | Vantage® Fixed-Bearing Polyethylene Liner Component | 1537 |
| Vantage® Mobile-Bearing Polyethylene Liner Component (TAR) | 761 | |
| 350-41-XX | Vantage® Mobile-Bearing Polyethylene Liner Component | 352 |
| 350-42-XX | Vantage® Mobile-Bearing Polyethylene Liner Component | 409 |